Ross Wilson

Assistant Adjunct Professor of Molecular Therapeutics
Lab Homepage: https://www.rosswilsonlab.org/Research Interests
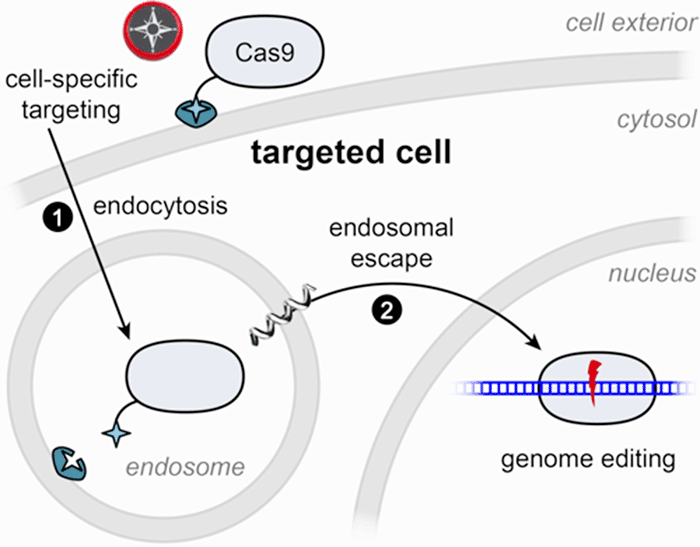
The Wilson lab focuses on engineering genome editing enzymes for cell-targeted delivery. CRISPR-based genome editing technology has rapidly transformed biomedical research and shows great promise for the development of novel therapeutic applications. Enzymes such as Cas9 (represented in the figure below) already contain several powerful properties: binding to a specific region of the genome and performing a precise cut at that site (left, below). This provides the foundation for therapies that may soon be able to correct the genetic defects that give rise to disease. But for this promise to be fully realized, therapeutic enzymes must be delivered to cells safely and efficiently. Our laboratory is working to develop genome-editing enzymes that are readily internalized by cells (bottom right, below). Furthermore, we strive to perform targeted delivery of these enzymes, maximizing precision in correcting specific cells, tissues, or organs (top right, below). A first step towards realizing this approach has been published in Rouet et al. JACS 2018 (see summary below, under Publications).

Recent advances in genetic and cell-based technology have demonstrated the transformative power of next-generation therapeutics. However, these treatments are incredibly expensive, and are often only available at a handful of cutting-edge research hospitals. Tragically, there are millions of people in the developing world who would benefit from such treatments, but deployment is currently impractical. The Wilson Lab’s efforts are motivated by the pressing need to improve access to life-changing technologies. A central goal of our work is to find affordable and straightforward new ways to genetic medicines, enabling cures globally.
Current Projects
Liver-targeted delivery
To direct Cas9 to liver cells, we rely on a receptor that is distinct to hepatocytes, the key functional cells of the liver. By tethering Cas9 to a small molecule that is recognized by the liver-specific receptor, we can induce efficient uptake of Cas9 into the cells. However, this represents only half of the journey to an edited cell: if no further action is taken, the Cas9 will move from endosomal cellular compartments to be digested in the cell’s lysosomes. To help Cas9 complete its journey, we include chemicals that promote endosomal escape. Ongoing efforts focus on tethering those chemicals to the surface of Cas9, giving it all the properties needed for a self-contained delivery and editing platform.
Delivery targeted to T cells or hematopoietic stem cells
Because of the proven clinical power of immune cell-derived therapies, we are interested in engineering Cas9 to target T cells for therapeutic editing. Furthermore, Cas9 has recently been used to cure hemoglobinopathies (e.g. sickle-cell disease) following genome editing of the patient’s hematopoietic stem cells. These two therapeutic approaches have thus far relied on ex vivo editing, which involves removal of the patient’s cells, modification of the cells in a laboratory setting, and return of the cells to the patient. In contrast, we hope to enable in vivo editing of a patient’s cells within the body, following a simple intravenous administration. Using a strategy analogous to the liver-targeted approach described above, we tether Cas9 to molecules that can specifically recognize T cells or stem cells, driving selective delivery even in the presence of other white blood cells. After achieving reliable targeting of a given cell type, we work to enable endosomal escape and efficient genome editing.
Improved genome editing in the brain or the lung
The Wilson lab is also involved in collaborative efforts to improve the delivery of CRISPR-derived enzymes (e.g. Cas9) to tissues such as the brain and the surface of the lung. Pre-formed Cas9 enzymes have demonstrated promise in editing these organs in vivo, but we hypothesize that their efficiency could be improved by engineering the enzyme to be more amenable to diffusion throughout the brain or through the mucus coating the surface of the lung.
Selected Publications
Foss, D. V. et al. Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes. Nat. Biomed. Eng. (2023). [link] [PMC]
Saha, K. et al. The NIH Somatic Cell Genome Editing program. Nature. (2021). [link] [PMC]
He, M. et al. A traceless linker for aliphatic amines that rapidly and quantitatively fragments after reduction. Chem. Sci. (2020).
Wilson, R. C. & Carroll, D. The Daunting Economics of Therapeutic Genome Editing. CRISPR J (2019).
Foss, D. V., Hochstrasser, M. L. & Wilson, R. C. Clinical applications of CRISPR-based genome editing and diagnostics. Transfusion (2019).
Rouet, R., de Oñate, L., Li, J., Murthy, N. & Wilson, R. C. Engineering CRISPR-Cas9 RNA-Protein Complexes for Improved Function and Delivery. CRISPR J (2018).
Rouet, R. et al. Receptor-Mediated Delivery of CRISPR-Cas9 Endonuclease for Cell-Type-Specific Gene Editing. J. Am. Chem. Soc. (2018).
Wilson, R. C. & Gilbert, L. A. The Promise and Challenge of In Vivo Delivery for Genome Therapeutics. ACS Chem. Biol. (2018).
Foss, D. V. & Wilson, R. C. Emerging Strategies for Genome Editing in the Brain. Trends Mol Med (2018).
Last Updated 2024-08-25