Helen Bateup

Associate Professor of Molecular Therapeutics
Lab Homepage: https://bateuplab.berkeley.eduResearch Interests
Neurons dynamically modulate their synaptic inputs in a variety of ways to facilitate learning and storage of new information. At the same time, specialized mechanisms are in place to maintain excitability and firing within a bounded range to preserve balanced activity in neural circuits. Notably, alterations in this delicate balance are thought to contribute to the manifestations of neurological and psychiatric disease. The goal of my laboratory is to understand the molecular mechanisms by which neurons modulate synaptic, neuronal, and network excitability. In particular, we are interested in how mutations in genes associated with disorders such as epilepsy and autism lead to altered nerual development, synapse and circuit function. To investigate this, we are taking a multi-disciplinary approach incorporating molecular, biochemical, imaging, electrophysiological, and behavioral analyses in mouse models and human stem cell-derived brain organoids. Through this diversity of approaches we hope to understand how molecular and genetic events change cellular and network function to ultimately impact behavior.
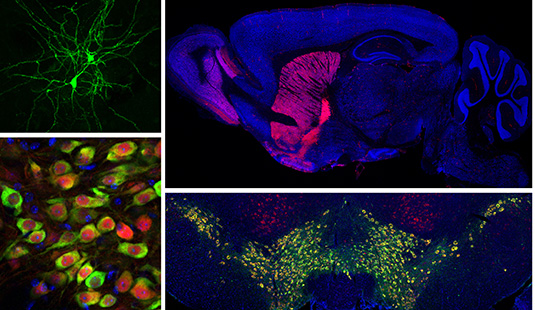
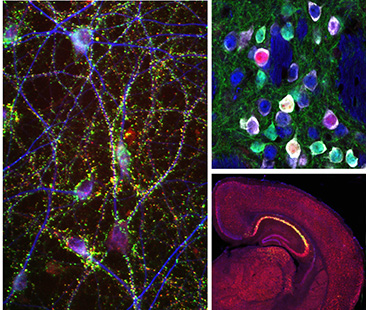
Current Projects
Basal ganglia dysfunction in autism spectrum disorders
The basal ganglia are a group of sub-cortical brain structures responsible for integrating sensory and motivational information to select and learn appropriate actions. We are studying how dysfunciton of the basal ganglia contributes to the behavioral manifestations of autism spectrum disorder (ASD). To investigate this, we use slice electrophysiology and behavior tasks in genetic mouse models of ASD to determine the relevant circuits, cells and synapses that may be comprimised in autism.
Genetically defined human neuron models of neurodevelopmental disorders
Recent advances in cellular reprograming and genome editing have endabled the generation of genetically engineered human cells for in vitro disease modeling. We have leveraged these approaches to generate a human neuronal model for the neurodevelopmental disorder Tuberous Sclerosis Complex (TSC), caused by mutations in the mTOR regulators TSC1 and TSC2. Our goal is to determine how mutations in the TSC genes impact human neuronal development and function.
Unraveling the complexity of neuronal mTOR signaling
The mTOR pathway is a central signaling hub that integrates intra- and extracellular signals to control processes related to cell growth and metabolism. Mutations in components of the mTOR pathway lead to syndromic neurodevelopmental disroders including TSC, which are often associated with ASD, epilepsy, and intellectual disability. It is not well understood how deregulated mTOR activity leads to neurological and psychiatric dysfunction. To investigate this, we use molecular profiling and biochemical approaches in mouse and human neurons to define the up- and down-stream components of the mTOR pathway. In addition, we are using imaging and electrophysiology to determine the functional impact of mTOR signaling perturbations, and test approaches to restore balanced mTOR activity in the context of disease.
Selected Publications
Blair, J.D. and Bateup, H.S. (2020) New frontiers in modeling tuberous sclerosis with human stem cell-derived neurons and brain organoids. Developmental Dynamics, Jan;249(1):46-55.
Kosillo, P., Doig, N.M., Ahmed, K.M., Agopyan-Miu, A.H.C.W., Wong, C.D., Conyers, L., Threlfell, S., Magill, P.J., and Bateup, H.S. (2019) Tsc1-mTORC1 signaling controls striatal dopamine release and cognitive flexibility. Nature Communications, Nov 28;10(1):5426.
Blair, J.D., Hockemeyer, D., and Bateup, H.S. (2018) Genetically engineered human cortical spheroid models of tuberous sclerosis. Nature Medicine, Oct 8;24(10), 1568-1578.
Kramer, D.J., Risso, D., Kosillo, P., Ngai, J., and Bateup, H.S. (2018) Combinatorial expression of Grp and Neurod6 defines dopamine neuron populations with distinct projection patterns and disease vulnerability. eNeuro, Jun 13;5(3), pii: ENEURO.0152-18.2018.
Benthall, K.N., Ong, S.L., and Bateup, H.S. (2018) Corticostriatal Transmission is selectively enhanced in striatonigral neurons with postnatal loss of Tsc1. Cell Reports, Jun12;23(11), 3197-3208.
Yang, H., Jong, J.W., Tak, Y., Peck, J., Bateup, H.S., and Lammel, S. (2018) Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron, Jan 17;97(2), 434-449.
Blair, J.D., Hockemeyer, D., Doudna, J.A., Bateup, H.S., and Floor, S.N. (2017) Widespread translational remodeling during human neuronal differentiation. Cell Reports, Nov 14;21(7), 2005-2016.
Kulkarni, R.U., Kramer, D.J., Pourmandi, N., Karbasi, K., Bateup, H.S., and Miller, E.W. (2017) Voltage-sensitive rhodol with enhanced two-photon brightness. PNAS, Mar 14;114(11), 2813-2818.
Blair, J.D., Bateup, H.S., and Hockemeyer, D.F. (2016) Establishment of Genome-edited Human Pluripotent Stem Cell Lines: From Targeting to Isolation. J Vis Exp, Feb 2;(108):e53583.
Photo credit: Mark Hanson at Mark Joseph Studios.
Last Updated 2020-09-10